Research: Dual-Targeting Strategy Shows Promise in Halting Melanoma Metastasis by Disrupting the Extracellular Matrix
New research reveals a dual-targeting approach that inhibits LOX and HSP70 — key drivers of extracellular matrix remodeling — to suppress melanoma metastasis and enhance immunotherapy response.
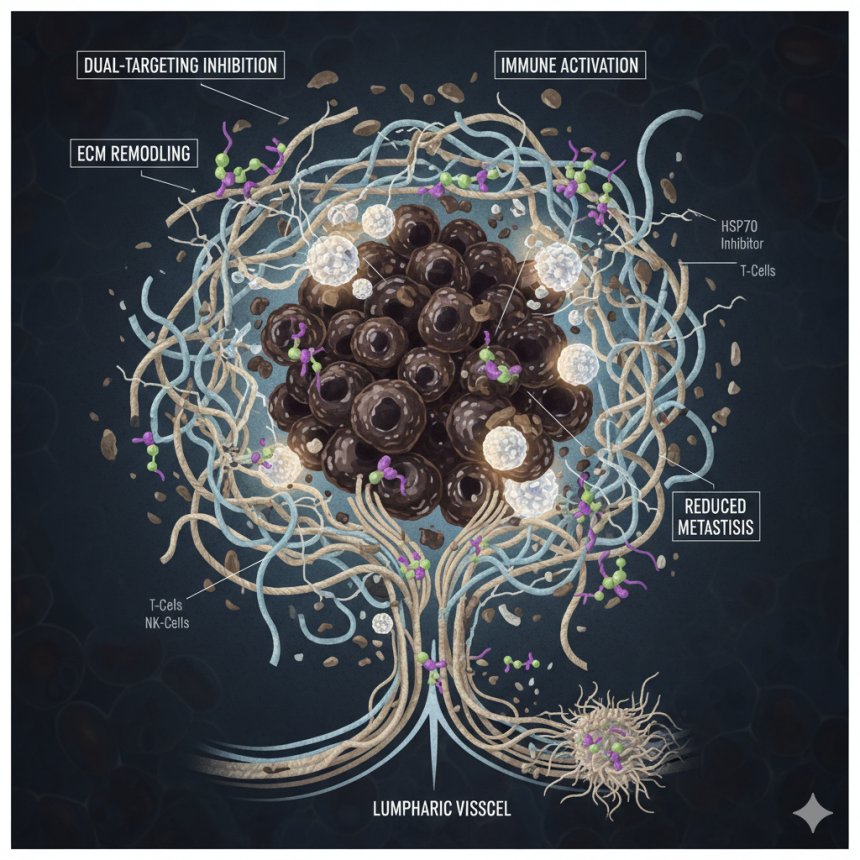
Introduction
The extracellular matrix (ECM) — a complex network of proteins that provides structural and biochemical support to cells — plays a central role in the progression and metastasis of solid tumors. In many cancers, including melanoma, the ECM not only provides physical scaffolding but also actively drives tumor growth, invasion, and immune evasion. Despite its critical role, ECM-targeted therapies have remained a largely untapped frontier in oncology.
In groundbreaking new research, scientists have unveiled a dual-targeting strategy that attacks melanoma’s ECM support system using engineered bispecific agents designed to inhibit lysyl oxidase (LOX) and heat shock protein 70 (HSP70) — two major players in melanoma progression.
A Dual-Targeting Breakthrough: LOX and HSP70
Building upon the inhibitory functions of lysyl oxidase–propeptide (LOX-PP), the research team designed biselective decoy molecules capable of binding and neutralizing both LOX and HSP70 simultaneously.
-
LOX is a collagen cross-linking enzyme that strengthens the tumor ECM, facilitating cancer invasion and metastasis.
-
HSP70, a molecular chaperone, protects cancer cells under stress, aiding their survival and promoting resistance to therapies.
By combining inhibition of these two proteins, the researchers aimed to break down melanoma’s structural and biochemical defenses.
Key Findings in Melanoma Models
When tested in mouse models of melanoma, this dual-targeting decoy therapy demonstrated remarkable effects:
-
Reduced Tumor Growth: The decoys significantly decreased tumor burden and the number of circulating melanoma cells.
-
Inhibited Metastasis: Lung metastasis was notably reduced, showing strong anti-proliferative activity.
-
ECM Remodeling Disruption: The decoys dismantled cancer-supporting ECM organization and suppressed remodeling enzymes.
-
Immune Microenvironment Reprogramming: Treatment enhanced infiltration of neutrophils, B cells, and CD8+ T cells, creating a more immune-responsive tumor environment.
When combined with immune checkpoint inhibitors, the dual decoys amplified CD8+ T-cell activity, further improving melanoma cell killing.
Ex Vivo Success and Translational Potential
Beyond animal studies, the engineered decoys bound effectively to multiple human tumors expressing LOX+/HSP70+, highlighting their potential in a broad range of solid malignancies beyond melanoma.
This cross-species efficacy positions dual LOX/HSP70 inhibition as a promising therapeutic avenue not only for melanoma but also for other ECM-driven cancers, such as breast, pancreatic, and lung carcinomas.
The Bigger Picture: Remodeling the Tumor Ecosystem
This study underscores a growing shift in cancer therapy — from focusing solely on tumor cells to reprogramming the tumor microenvironment. By dismantling the ECM’s pro-cancer architecture, the dual-targeting decoys expose the tumor to immune attack and reduce metastatic potential.
The approach also opens pathways for combination therapies that integrate ECM disruption with checkpoint blockade, targeted inhibitors, or CAR-T immunotherapy, potentially overcoming current resistance mechanisms.
Conclusion
The discovery of bispecific LOX/HSP70-targeting decoys represents a major advancement in the fight against melanoma. By attacking both structural (ECM) and molecular (HSP70) supports, this dual-inhibition strategy not only suppresses metastasis but also boosts immune response — offering new hope for ECM-targeted immunotherapy.
Future clinical trials will be crucial in determining its translational impact, but the findings already provide a compelling framework for next-generation cancer treatments that remodel the tumor’s physical and immune landscape simultaneously.
References
-
Zhang, J. et al. (2025). Dual targeting of LOX and HSP70 disrupts extracellular matrix remodeling and enhances immunotherapy in melanoma. Nature Cancer, 6(10), 1243–1258.
-
Cox, T. R. & Erler, J. T. (2023). Remodeling and homeostasis of the extracellular matrix: Implications for cancer therapy. Trends in Cell Biology, 33(2), 123–136.
-
Schaefer, L. (2024). Targeting the extracellular matrix in cancer therapy. Frontiers in Oncology, 14, 1194021.
-
Jayaprakash, P. et al. (2025). Heat shock proteins and tumor microenvironment: Modulators of cancer immunity. Cancer Immunology Research, 13(4), 711–725.


















