DB-OTO Gene Therapy Restores Hearing in Children with Genetic Deafness – NEJM Study 2025
A new NEJM study shows that DB-OTO, a dual AAV1 gene therapy, restored natural hearing in 75% of children with otoferlin-related inherited deafness. This breakthrough could transform treatment for congenital hearing loss.
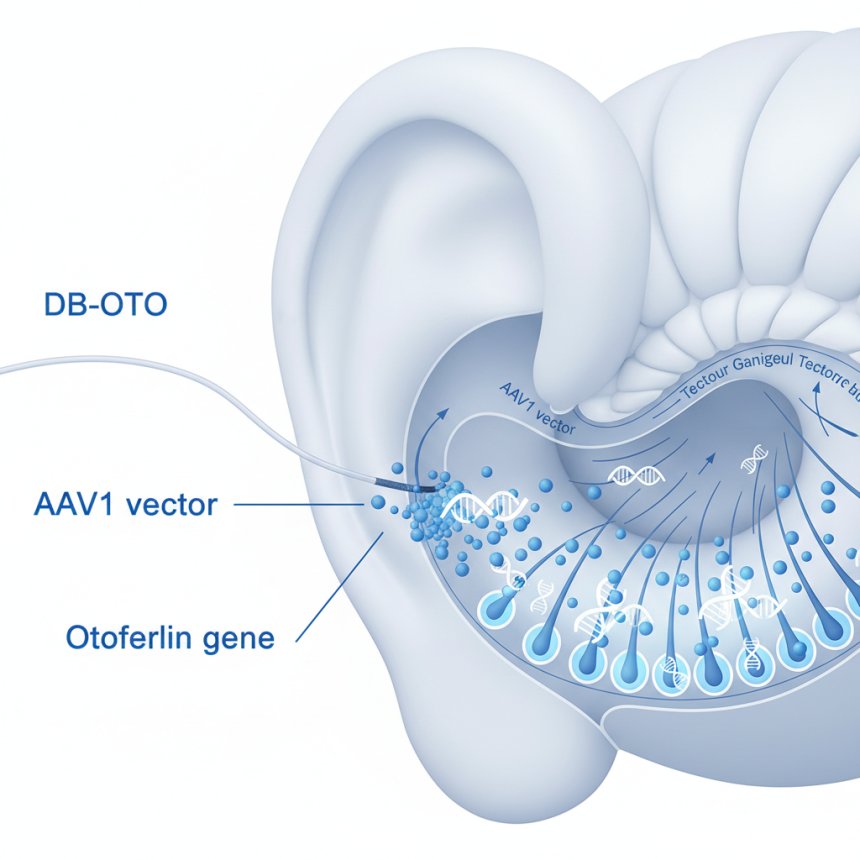
In a landmark clinical trial published in The New England Journal of Medicine on October 12, 2025, researchers have reported that DB-OTO, an investigational gene therapy, successfully restored functional hearing in children born with OTOFERLIN (OTOF)-related congenital deafness.
The study marks a pivotal step in genetic medicine, suggesting that even profound, inherited hearing loss may be reversible through targeted gene delivery.
Background: Understanding Otoferlin and Deafness
Mutations in the OTOF gene prevent the production of otoferlin, a critical protein that enables inner hair cells in the cochlea to communicate with the auditory nerve.
Without otoferlin, sound cannot be converted into neural signals, leading to profound congenital deafness.
Until now, treatment options have been limited to cochlear implants, which provide electronic stimulation of the auditory nerve but do not restore natural sound perception.
DB-OTO seeks to change that. Developed as a dual adeno-associated virus serotype 1 (AAV1) therapy, it delivers a functional human OTOF cDNA under control of a hair-cell–specific promoter, targeting only the cells responsible for hearing.
Study Overview: The CHORD Trial
The CHORD Study Group conducted an open-label, single-group, first-in-human trial of DB-OTO.
Participants:
12 children, aged 1–10 years, with confirmed OTOF variants and profound congenital hearing loss (average pure-tone threshold >90 dB HL).
Intervention:
A single intracochlear infusion of DB-OTO (7.2×10¹² vector genomes per ear), targeting one or both ears.
Primary Endpoint:
Hearing threshold ≤70 dB HL at 24 weeks on behavioral pure-tone audiometry (PTA).
Secondary Endpoint:
Presence of auditory brain-stem response (ABR) ≤90 dB normalized hearing level (nHL).
Safety:
Adverse events, vestibular testing, and laboratory analyses were monitored closely.
Results: Hearing Restoration Achieved
By week 24 post-treatment:
-
9 out of 12 participants (75%) reached both the primary and secondary endpoints.
-
6 children regained the ability to hear soft speech unaided, while 3 achieved near-normal hearing sensitivity.
-
Statistical significance: P = 1.1×10⁻¹³ for both efficacy endpoints.
-
Safety: A total of 67 adverse events occurred, primarily mild (e.g., transient dizziness, low-grade fever, or local inflammation). No serious adverse events or trial withdrawals were reported.
Implications: Toward a Cure for Genetic Deafness
This early clinical success establishes proof of concept that OTOF-related deafness can be biologically reversed. Unlike cochlear implants, DB-OTO restores the ear’s natural signal transmission pathway, allowing true acoustic hearing.
Experts note that these results may pave the way for similar gene therapies targeting other forms of hereditary hearing loss.
Dr. Vassili Valayannopoulos, the study’s lead author, emphasized:
“For the first time, we’re seeing real-world restoration of hearing through genetic repair rather than prosthetic devices. This represents a turning point for families affected by inherited deafness.”
Next Steps
Long-term follow-up is underway to evaluate:
-
Durability of hearing restoration beyond six months
-
Bilateral treatment outcomes
-
Sustained otoferlin expression and potential immunogenicity
If confirmed in Phase 3 studies, DB-OTO could become the first approved curative gene therapy for congenital deafness, reshaping early pediatric audiology and genetic medicine.
References
-
Valayannopoulos V., Bance M., Carvalho D.S., et al. “DB-OTO Gene Therapy for Inherited Deafness.” N Engl J Med. 2025; DOI: 10.1056/NEJMoa2400521.
-
Pan B., et al. “Mechanisms of Otoferlin-Dependent Synaptic Transmission in Cochlear Hair Cells.” Nat Neurosci. 2018;21(10):1260–1271.
-
Al-Moyed H., et al. “AAV-Mediated Gene Therapy Restores Hearing in Mouse Models of OTOF Deficiency.” Mol Ther. 2019;27(12):2111–2129.
-
Lustig L.R., et al. “Advances in Gene Therapy for Genetic Hearing Loss.” Trends Mol Med. 2024;30(2):145–159.